![[Image: Ultimaker]](https://fabbaloo.com/wp-content/uploads/2020/05/image-asset_img_5eb0a1e67a208.jpg)
Materialise’s announcement today of FDA-cleared medical model 3D printing encompasses vat polymerization and material extrusion technologies in addition to PolyJet.
At RSNA, the company announced work with Stratasys toward FDA-cleared medical 3D printing and it turns out that’s not the whole story: Materialise’s new program extends to Ultimaker and Formlabs for, respectively, the S5 and Form 2 3D printers.
Materialise explains:
“The Mimics inPrint Certification Program is designed to identify compatible printers and materials, so you can start your 3D printing facility at the point-of-care with both hardware and software fully certified for diagnostic applications.”
With Mimics inPrint the only software certified for clinical applications for 3D printing, Materialise is well positioned to collaborate — a speciality for the company. Setting up an in-house 3D printing lab requires significant resources, and in a healthcare environment that includes ensuring that all medical needs and regulations are met. Having FDA-certified software working with validated hardware and materials smooths the path for healthcare providers to do just that: provide healthcare.
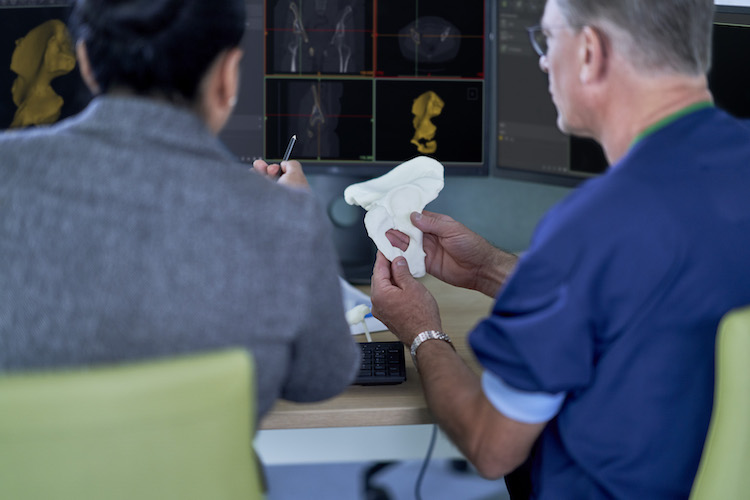
Medical models, 3D printed to exactly match a patient’s specific anatomy, offer unprecedented hands-on access to the exact anatomy in consideration for a given diagnosis or procedure.
“Our goal is to help you make your 3D lab efficient, innovation-driven and ultimately patient-centric. Towards that goal, we undertook thorough testing in collaboration with industry-leading 3D printer manufacturers. Performance testing included a rigorous testing and analysis of specific material-printer combinations to determine whether, when adhering to the technical instructions from the machine manufacturer, models were printed accurately compared to specific acceptance criteria for each clinical indication,” Materialise says.
The full grouping as of today for these partners includes Stratasys’ J750 and J735, Object30 Prime, and Connex 2 500 with a variety of materials; Formlabs’ Form 2 with Clear FLGGPCL02 material; and the dual-extrusion Ultimaker S5 with PLA.
Working with three different 3D printing technologies from three different well-known suppliers highlights Materialise’s hardware-agnostic approach to finding a collaborative best solution for its customers.
“More and more frequently, doctors look to 3D printing for pre-operative planning and the fabrication of physical models for patient management, treatment and surgeon-to-surgeon communications,” said John Kawola, President, Ultimaker North America. “As diagnostic usage of 3D printers continues to revolutionize patient care, safety and quality remain a top concern for hospitals.The Materialise certification of the 3D printing workflow when used with Mimics inPrint reduces the safety- and quality-control burden on doctors and hospitals with it’s clearance by the FDA.”
Desktop solutions based on extrusion and SLA technologies allow for more options — at a more convenient price point for many 3D labs — to create medical models.
“Formlabs Form 2 was the first and remains the only stereolithography 3D printer to be validated by Materialise as part of the first FDA-cleared process to create accurate anatomical models for diagnostic use. Having the most affordable validated printer and one with biocompatible materials, we’ve been fortunate enough to work with industry leading hospitals and clinicians for years. We hope that the validation of additional printers will lead to increased clinical adoption and to enhancements in the standard of care,” Gaurav Manchanda, Director of Healthcare at Formlabs, said. “It’s an exciting time in the healthcare space and we’re looking forward to deepening our collaboration with Materialise. This is an exciting step towards our vision of better planning for surgeons, better outcomes for patients, and a better bottom line for healthcare systems.”
The three partners introduced today offer an excellent start to the new certification program — and, indeed, it is a start. Materialise notes that they “continue to work with hardware manufacturers to test more printers and ultimately increase the pool of certified tools available.”
![Workflow via the Materialise Mimics inPrint Certification Program [Image: Formlabs]](https://fabbaloo.com/wp-content/uploads/2020/05/materialise-formlabs_medical-models-3d-printing-workflow.png.1354x0_q80_crop-smart_img_5eb0a1e6e2e41.png)
Materialise lists as benefits of the Mimics inPrint Certification Program:
-
Solid foundation
-
Start your hospital 3D lab on a solid foundation with the world’s first and only 3D printing software certified for clinical use, Mimics inPrint, and fully compatible hardware
-
-
Less overhead
-
Save time and reduce the burden on your quality control processes by starting off with pre-vetted printers and materials
-
-
Credibility
-
Build internal and external credibility for your new 3D lab by using certified tools and materials
-
It bears repeating that collaborative efforts such as this are driving the industry not just to a better place — but to one set to do more tangible good for actual people.
RSNA continues this week in Chicago; Materialise is located in booth 4114, and in the 3D Printing & Advanced Visualization Showcase will be in1968K5 with Ultimaker in booth 1968J and Formlabs in booth 1968D.
Via Materialise, Ultimaker, and Formlabs
[Updated to add perspective from Formlabs 11/26/18]










FELIXprinters has released a new bioprinter, the FELIX BIOprinter, which is quite a change for the long-time 3D printer manufacturer.