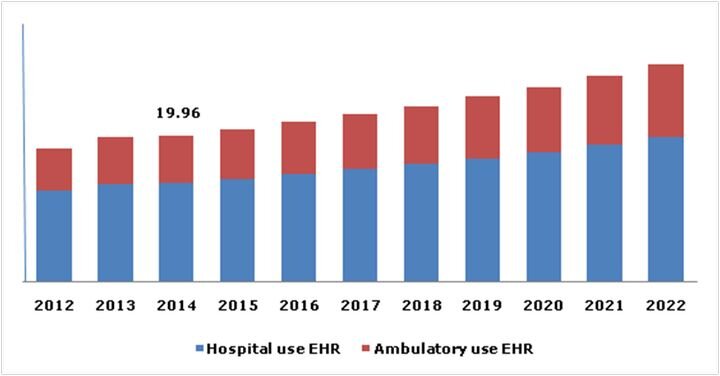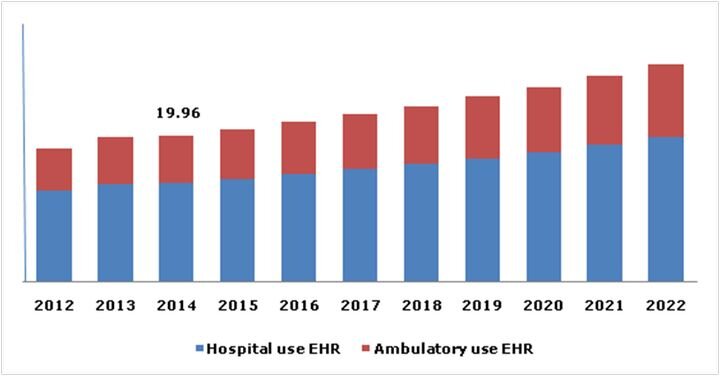
Charles Goulding and Preeti Sulibhavi review the possibilities of data privacy concerns slowing the advancement of 3D printing in the healthcare market.
Concerns about privacy related to patient medical data have recently been front-page news. We all cherish our right to privacy. However, data mining of patient data is an undeniable pathway to major medical breakthroughs that can benefit everyone. When the long-anticipated human genome was finally mapped, the excitement soon ebbed when scientists realized that we didn’t have the corresponding patient disease data to benefit from this magnificent accomplishment.
Now the amount of electronic medical patient data has vastly grown, we can begin to benefit from genome and disease data matching. Medical device designers and manufacturers are increasingly using 3D printing for new innovative solutions including 3D bioprinting, embedded electronic medical devices, orthopedics and a myriad of other health and disease conditions.
The doctors, technicians and product designers who want to address these important needs based on priorities and opportunities can’t progress efficiently without large volumes of patient data. The 3D printing industry needs to insert itself into the critical Privacy vs. Patient Data debate. Unless the 3D printing industry makes its case it may be marginalized by well-meaning privacy advocates who often don’t weigh the medical innovation upside.
Research and Development tax credits are available to support innovation related to enhanced data protection and the resulting medical devices that will improve patient health outcomes.
For example, it can’t be determined whether a particular 3D printer knee joint is effective and has the expected useful life intended unless there is relevant post-operative patient data. Many patients are willing to waive privacy for the benefit of themselves, their families and mankind and they should be afforded the right to do so.
In December 2019, Mark Barnes received the award for Innovator-of-the-Year from the Financial Times. In 2018, Mark Barnes co-founded Vivli, a non-profit organization that promotes the sharing of data from clinical research trials. According to Barnes, “We have two worlds in collision – on the one hand, with the promulgation and proliferation and uses of big data, is the idea of transparency and that data should be used and deployed and should bring good and bring profit; on the other, we have the push towards privacy with people saying they own their data and want to control their data.” Barnes is described as “on a mission to share medical data.”
The Research & Development Tax Credit
Enacted in 1981, the now permanent Federal Research and Development (R&D) Tax Credit allows a credit that typically ranges from 4%-7% of eligible spending for new and improved products and processes. Qualified research must meet the following four criteria:
-
Must be technological in nature
-
Must be a component of the taxpayer’s business
-
Must represent R&D in the experimental sense and generally includes all such costs related to the development or improvement of a product or process
-
Must eliminate uncertainty through a process of experimentation that considers one or more alternatives
Eligible costs include US employee wages, cost of supplies consumed in the R&D process, cost of pre-production testing, US contract research expenses, and certain costs associated with developing a patent.
On December 18, 2015, President Obama signed the PATH Act, making the R&D Tax Credit permanent. Beginning in 2016, the R&D credit can be used to offset Alternative Minimum Tax for companies with revenue below $50MM and, startup businesses can obtain up to $250,000 per year in cash rebates applied toward payroll taxes.
A Healthy Balance
The medical data privacy issue is at the forefront and the time to participate in the public debate is now. 3D printing is finally mainstreaming and can help drive medical innovation but it must emphasize the importance of data analysis in the medical innovation process while considering privacy concerns.