Charles R. Goulding and Arianna Coger listen to ways 3D printing continues to innovate in hearing aid production.
Hearing loss affects a significant percentage of our population, with approximately 15% of American adults reporting hearing troubles. With the invention of headphones, the number of adults with hearing loss is expected to drastically increase in the coming years. According to the National Institute on Deafness and Other Communication Disorders, approximately 28.8 million U.S. adults could benefit from using hearing aids but do not use them. This is partially due to factors such as high costs and government policy. The New York Times article entitled “Hearing Aids for the Masses” discusses how hearing aids are often expensive, costing thousands of dollars for a pair, and require a prescription from an audiologist. Additionally, hearing aids are often not covered by healthcare insurance. As a result, few people in need of hearing aids can easily access them.
The Struggle for Over-the-Counter Hearing Aid FDA Approval
The FDA has yet to release regulations to define safety and effectiveness benchmarks for over-the-counter (OTC) hearing aids.
On July 9th, 2021, President Biden signed an executive order calling for the Health and Human Services to develop rules for allowing OTC hearing aids within 120 days. The FDA is a subdivision of the Health and Human Services regulating all medical pharmaceuticals and devices. Currently, these OTC devices cannot legally be called hearing aids; instead, they are often referred to as personal sound amplification products (PSAPs). There are currently many unregulated PSAPs on the market without defined standards that can be used to verify whether they are truly safe and effective. As a result, many consumers find it difficult to believe OTC devices are reliable or they may purchase cheap OTC devices that do not work well. With FDA approval, the quality of these devices could be validated, and they could be used as a convenient alternative to expensive prescription hearing aids.
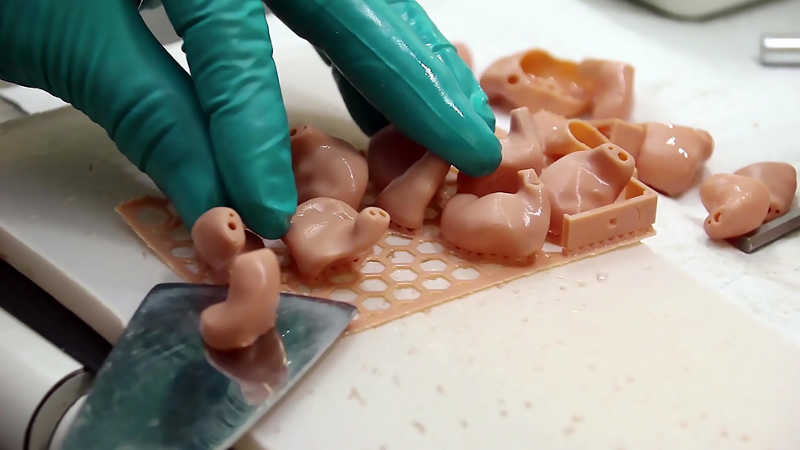
3D Printed Hearing Aids
Hearing care providers like Phonak and GN Resound have used 3D printers from EnvisionTEC to manufacture custom-designed hearing aids for the past 5-10 years. However, the hearing aids they manufacture cost several thousand dollars. This is partially due to the high cost of industrial 3D printers as well as the constant research and development performed to reduce the size and increase comfort.
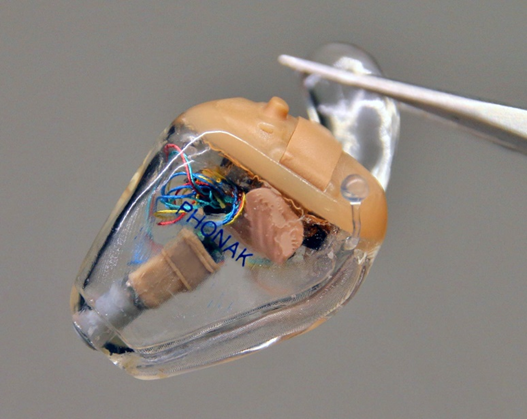
Both of the companies mentioned manufacture FDA-approved prescription hearing aids. OTC hearing aids are currently available but not marketed as medical devices due to the lack of FDA infrastructure for approval. Multiple companies and organizations have tried to develop cheaper alternatives to prescription hearing aids.
Researchers from Georgia Tech were able to develop a prototype OTC device called LoChAid with the components encased in a 3D printed enclosure. The materials for LoChAid would cost less than $1 when purchased in bulk while allowing personalization through 3D printing.
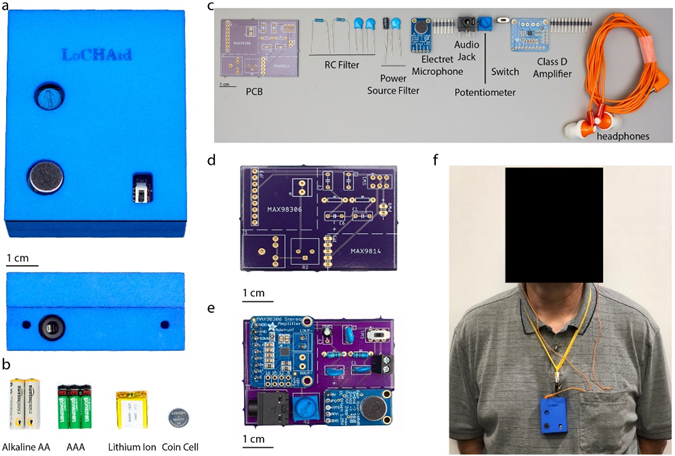
Companies taking on similar endeavors may be eligible for Research and Development Tax Credits.
The Research & Development Tax Credit
The now permanent Research and Development (R&D) Tax Credit is available for companies developing new or improved products, processes and/or software. As of 2016, eligible startup businesses can use the R&D Tax Credit against $250,000 per year in payroll taxes.
3D printing can help boost a company’s R&D Tax Credits. Wages for technical employees creating, testing, and revising 3D printed prototypes can be included as a percentage of eligible time spent for the R&D Tax Credit. Similarly, when used as a method of improving a process, time spent integrating 3D printing hardware and software counts as an eligible activity. Lastly, when used for modeling and preproduction, the costs of filaments consumed during the development process may also be recovered.
Whether it is used for creating and testing prototypes or for final production, 3D printing is a great indicator that R&D Credit eligible activities are taking place. Companies implementing this technology at any point should consider taking advantage of R&D Tax Credits.
Conclusion
Pressure for the FDA to regulate OTC hearing aid products continues to increase as more people desire affordable solutions for hearing loss. As the average life expectancy of our population continues to grow, we will need better cost-efficient solutions to improve quality of life for our senior citizens.
