
[Source: Wikimedia]
Andressa Bonafe and Charles Goulding interview a leading researcher to learn more about the state of bioprinting.
We at R&D Tax Savers have been following with great enthusiasm the developments related to 3D bioprinting around the world.
We recently became aware of the impressive work carried out by Brazilian researchers who created a protocol for 3D printing brain cells. Curious to learn more about those involved in this initiative, we interviewed Bruna Alice Gomes de Melo, first author of the article published in the Journal of Visualized Experiments (JoVE) in July 2021. She gave us her perspective on the current 3D bioprinting landscape and told us about some of the projects she has been involved with.
Thank you for taking the time to speak with us. Could you tell us about your career as a researcher, how you got involved with 3D bioprinting, and why you chose to specialize in this technique?
I received a bachelor’s degree in chemistry, a master’s and a doctorate in chemical engineering at the University of Campinas (UNICAMP). My master’s degree was focused on nanoparticles, a topic that was on the rise at the time. During my PhD, my advisor specialized in regenerative medicine, an area that had always interested me. As a result, I started to study more about cells, biomaterials, and biofabrication techniques, the three pillars of bioengineering.
During the PhD, I received a scholarship from São Paulo Research Foundation (FAPESP), which opened the way for me to work for nine months in a bioengineering lab at Harvard Medical School, under the guidance of Dr. Su Ryon Shin. The research group was interdisciplinary, with people from chemistry, physics, medicine, and mechanical engineering. The lab was very strong in 3D bioprinting, a technique that was essential to several of the projects in which I was involved and that eventually resulted in publications.
This experience with 3D bioprinting and regenerative medicine led me to do a post-doctorate at the Federal University of São Paulo (UNIFESP), in a molecular neurobiology laboratory. Dr. Marimélia Porcionatto, who was responsible for the lab, sought to implement a more multidisciplinary team that would develop projects involving 3D bioprinting. With this mission, I submitted a grant application to FAPESP that eventually enabled the acquisition of a printer from 3DBS, a Brazilian startup. Later, another grant funded the purchase of a larger printer and materials that were utilized in the brain cells project.
Throughout this journey, my interest hasn’t been in a specific type of tissue, but rather in manufacturing and bioengineering techniques. I’ve worked with cartilage cells, bone, cardiac cells, neural tissues, etc.
What do you consider to be the biggest advantages of 3D bioprinting compared to other fabrication methods?
3D bioprinting enables the creation of complex structures, mimicking tissues that make up the human body. Through bioengineering, this technique allows for in vitro studies with living cells, which are a step prior to animal models. This is important because it leads to a reduction in the number of animals involved in research. With 3D bioprinting, we have the resources to evaluate the performance of drugs, vaccines, viruses, etc. with greater precision. 3D models are superior to 2D alternatives, in which cells are grown in plates, since tissues in the human body are three-dimensional. The morphological proximity is a great advantage. Even if compared to other methods of producing 3D structures, such as the use of molds, bioprinting allows the creation of more complex models, with different combinations of cells and biomaterials, which is possible, for example, through the use of more than one extruder nozzle. This advantage gives us the prospect of creating diverse tissues and, one day, even organs for implants.
You mentioned your interest in the manufacturing method itself. Does this involve biomaterials research?
Yes, an important aspect of 3D bioprinting research is the use of biomaterials. More specifically, we are talking about polymers and, for the most part, natural polymers, which are present in the human body, such as collagen. Hyaluronic acid, gelatin (hydrolyzed collagen), and even some synthetic polymers are also used. In general, they are softer and more hydrated polymers, with the exception of cases involving bones or prostheses, which require rigid materials such as ceramics. The difference between bioprinting and traditional 3D printing is that the materials are not subjected to such severe conditions, involving high temperatures. Still, we need detailed studies of their properties to determine their ability to mimic the tissues of interest. For example, in the projects I’ve been involved in, we wanted to determine whether the combination of collagen, gelatin, and fibrin would create conditions that are comparable to brain tissue mechanics.
In recent years, you have been involved in very interesting and diverse research. Could you give us an overview of the role of 3D bioprinting in some of these projects?
During my time at Harvard, I worked with mesenchymal stem cells and cartilage. Cartilage is an interesting area because it is a tissue that wears down with age and does not regenerate. It is also a less complex tissue if compared, for example, to neural tissues. Even so, throughout the project, the study of the materials was essential to create a suitable environment. It had to resemble cartilage in terms of rigidity, but at the same time, allow the mesenchymal stem cells to adapt and eventually differentiate into chondrocytes, which are cartilage cells. First, we used a mold to create the rigid fabric. Then, we mixed the stem cells in a softer material and, with the bioprinter, we printed this combination inside the rigid material. This technique allowed us to deposit the cells in the direction and arrangement we wanted. In this way, we created living tissue that had the rigidity of cartilage but also featured softer material around the cells. This configuration allowed the cells to differentiate into chondrocytes and express their genes, demonstrating behavior similar to that of cartilage. This work was published in the journal Advanced Functional Materials.
More recently, as a postdoctoral fellow, I did several studies of biomaterials to identify combinations and bioprint designs that would be best suited to mimic brain tissue. These were preliminary studies that will serve as a basis for future work focused on neurodegenerative diseases.
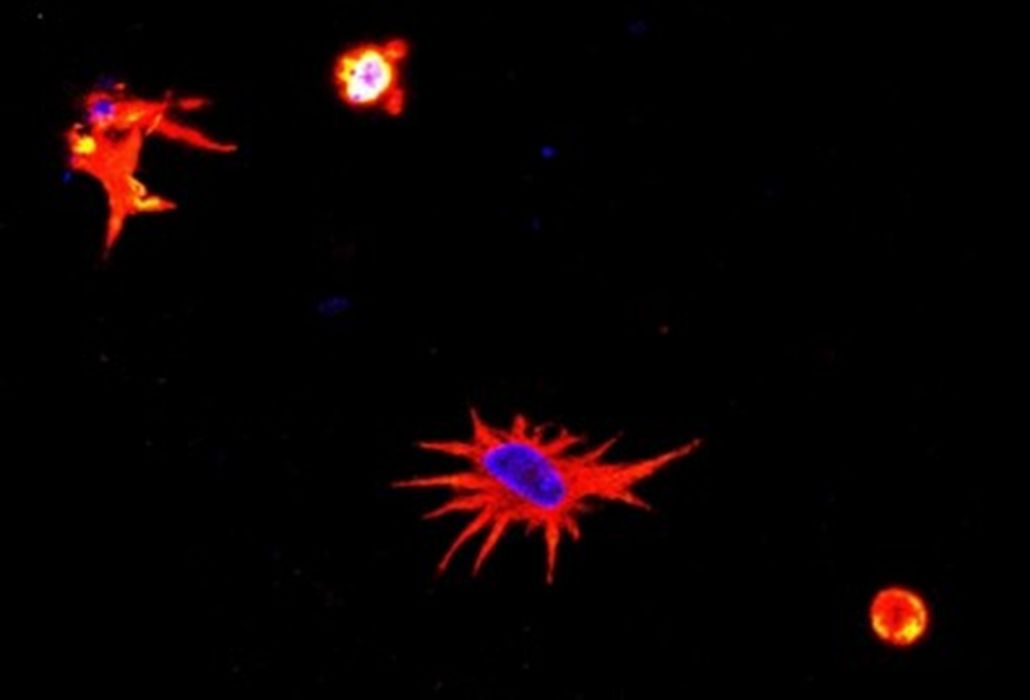
Another interesting project during my postdoc was the development of a model to evaluate the effects of SARS-CoV-2 on neural cells. To build this model, we mixed astrocytes and neurons, which are the main cells of neural tissue. First, we 3D bioprinted with astrocytes, which are more resistant, and then we added neurons. These cells connected and eventually recognized their environment as neural tissue. From this point forward, our objective was to observe the behavior of the cells in the presence of the virus. For example, we wanted to check whether there would be expression of inflammatory proteins, whether the morphology of the cells would undergo changes, etc. Another interesting aspect of the project is that we used animal cells, so we had to adapt the human virus to mice. This project, also financed by FAPESP, resulted in the publication of an article in the journal Advanced Biology.
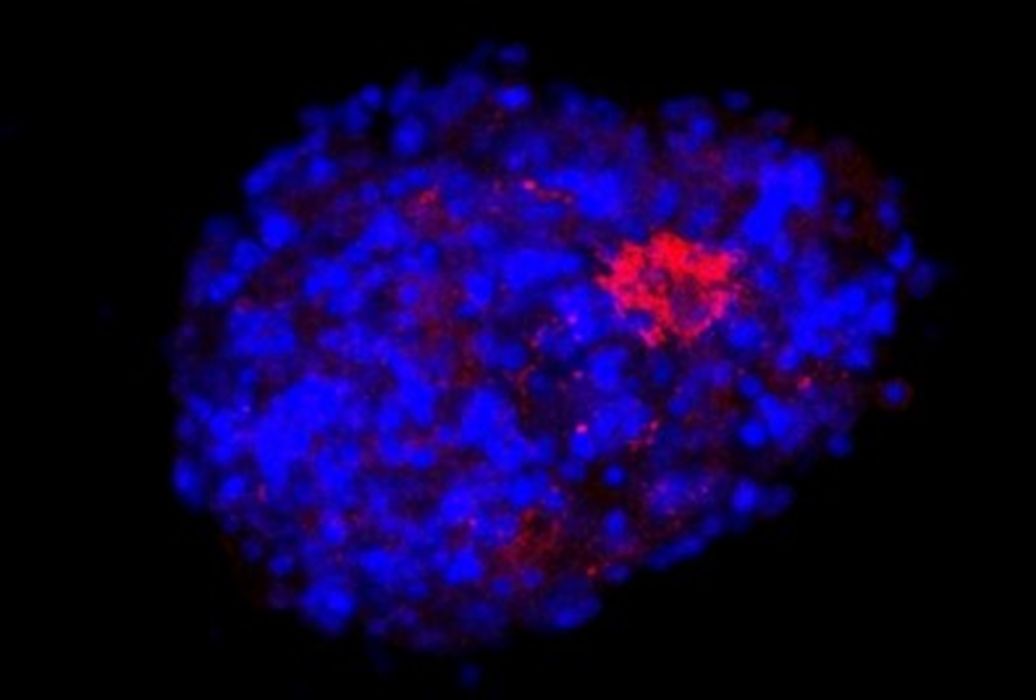
Currently, I work at the startup Nanobioplus, based in Campinas, Brazil, with focus on the research and development of products involving biomaterials. I am also a collaborator at the Shin Lab, at Harvard Medical School. I recently joined a large interdisciplinary project that involves researchers from different areas across Brazil. Called Model3D, this initiative is part of the National Institutes of Science and Technology program. The main idea is to use organoid models to study non-transmissible chronic diseases, such as neurodegenerative diseases, neurodevelopmental diseases, and cancer. The initiative has more than R$5.5 million in funding and will certainly use 3D bioprinting techniques to achieve its goals.
Thinking about the future of 3D bioprinting, what would be the main challenges and areas that you would highlight as most promising?
I believe that the main challenge is to develop functional organs. In most cases, they involve very complex tissues. In addition to simulating the extracellular matrix using biomaterials, we have to combine different types of cells, build blood and lymphatic vessels, etc. Creating complete and functional organs, suitable for transplantation, is the biggest dream and the biggest challenge for anyone working with bioprinting. We have been making progress towards this goal, creating and testing small pieces of tissue that will eventually fit together like jigsaw puzzles.
Promising areas in the short term would be the expansion of the use of bioprinted tissues composed of biomaterials in the regeneration of tissues and small lesions, in addition to bioprinting with human cells for diagnosing diseases, and testing new drugs, treatments, and vaccines.
