Researchers seem to have solved a major problem in bioprinting.
Bioprinting has been around for many years and typically takes this form:
- A “scaffold” in the desired geometry is 3D printed
- Living cells are deposited on the scaffold by 3D printing
- Cells divide and grow along the scaffold’s geometry
- Scaffold dissolves over time, leaving just the cells in the correct geometry
However, it turns out that there are plenty of issues with scaffolds. Cells typically have poor interaction with scaffolds, leading to correspondingly poor prints. Much of this is due to the relatively bad resolution of the 3D printed scaffolds, which is far larger than the dimensions of living cells.
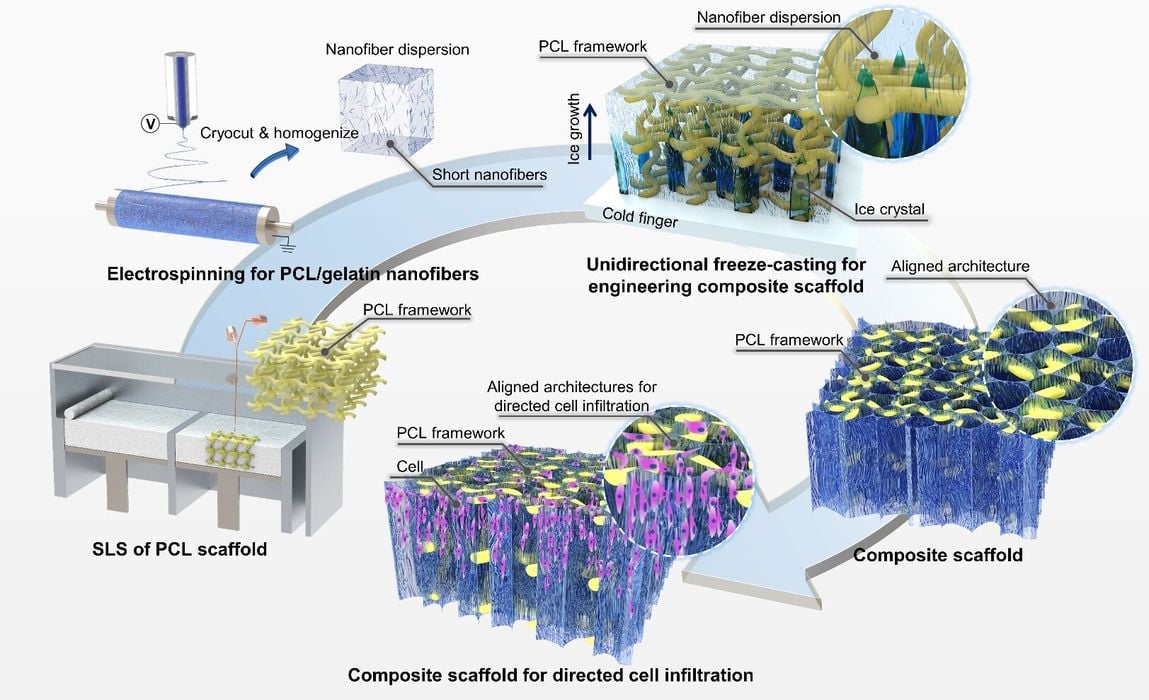
The researchers attempted to solve this problem by applying a number of different techniques to produce a new form of scaffold. They explain:
“Here, composite scaffolds with mechanically-robust frameworks and aligned nanofibrous architectures are presented and hybrid manufactured by combining techniques of 3D printing, electrospinning, and unidirectional freeze-casting.”
These approaches provided far better success for cells to integrate with the scaffold and even encouraged cellular division and growth.
“The composite scaffolds can provide volume-stable environments, enable directed cellular infiltration for tissue regeneration, support the adipogenic maturation of adipose-derived stem cells (ADSCs) in vitro, guide directed tissue infiltration and promote nearby neovascularization when implanted into a subcutaneous model of rats.”
A key technology used in the research was “electrospinning”, which is the creation of tiny fibers from electronhydrodynamic jetting. The fibers encouraged cell attachment and longer term survival.
Another unusual technology was “freeze casting”, which involves freezing infused liquids to trigger phase separation.
These technologies were combined with traditional FFF approaches to create a “composite” scaffold made using several methods. The result was effective, according to their paper:
“Here, we hybrid manufactured composite scaffolds with load-bearing frameworks and aligned nanofibrous architectures by combining techniques of 3D printing, electrospinning, and unidirectional freeze-casting for directed cell infiltration and tissue regeneration (figure 1). In the composite scaffolds, the 3D-printed frameworks provided sufficient mechanical strength for counteracting biological loads in vivo, while the embedded nanofibrous architectures with unidirectional micropores provided additional sites for cell adhesion. The effect of the pore size of the aligned nanofibrillar architectures on cellular attachment, proliferation, and directed infiltration was investigated. We also demonstrated the adipogenic maturation of the seeded adipose-derived stem cells (ADSCs) on the composite scaffolds in vitro. The efficacy of the hybrid manufactured composite scaffolds in guiding the ingrowth and penetration of the host soft tissues was further studied by a subcutaneous model of rats.”
This is a significant development in bioprinting, and one that could lead to new forms of bioprinting and opening up bio applications that have previously eluded success.
