
Charles R. Goulding and Johnson Jiang look at the partnership of Cellink and Lonza.
The Boston, U.S.-based company BICO and its Swedish subsidiary Cellink are offering more opportunities in the field of 3D bioprinting with help from the Swiss multinational Lonza Group AG. This partnership is part of a long list of developments in both the company’s history as well as in the bioprinting space.
Bioprinting At a Glance
The beginning of 3D printing (or additive manufacturing) technologies in 1984 sparked limitless opportunities in many industry sectors. However, the medical and microbiology communities were not ready to adopt 3D printers for their use. Until very recently, cell cultures could only be grown in 2D Petri dishes. But 1996 was the year when research demonstrated for the first time that biomaterials could be used in tissue regeneration. These findings motivated researchers to find novel pathways that could lead to regenerative medicines.
In 2001, researchers printed scaffolds of biological organs and seeded donor cells onto them. Then in 2003, Thomas Boland patented a method of ink-jet printing of cells with high survival rates. In 2004, the first tissue was directly printed without the need for a scaffold. This eventually led to the company Organovo selling the first commercially available 3D bioprinter, the Novogen MMX, in 2009. The availability of 3D bioprinters has since greatly expanded with many pharmaceutical and microbiology laboratories using them to better understand tissues, diseases, and potential therapeutic solutions.
The 3D bioprinting market was valued at US$771.41M in 2020 and is projected to grow to US$1.899B in 2025 with a CAGR of 19.4% as per the Business Research Company’s 2021 Bioprinting Report. With growing private and public interest in bioprinting research, the industry is expected to produce continued growth. The use of artificial intelligence (AI) has been a growing trend for many 3D bioprinting workflows. Current research is exploring AI data analysis capabilities in determining optimal printing parameters for biocompatible tissues based on patient physiology.
BICO / Cellink

Cellink was founded in 2016 and made headlines by being the first company to develop universal bioink that can be used in any 3D bioprinting setup. Cellink’s bioink product greatly improved the efficiency of research workflows globally. The company also introduced its own entry-level bioprinter INKREDIBLE priced for less than US$5,000 (a market low at the time).
Since then, the company has changed its name to BICO and has expanded its business model to support other areas of life science research. BICO still manufactures its bioprinter and bioink products under the Cellink name.
The company describes itself as a bioconvergence company, which integrates advanced digital technologies such as robotics and AI with microbiological research. The company provides cutting-edge technology solutions that enable life science research labs to carry out 3D bioprinting research. In 2020, BICO had an R&D expense-to-employee ratio of US$4,702 per employee.
Lonza
Lonza Group AG (Lonza) is a contract-development manufacturing organization (CDMO) founded in 1897 that provides manufacturing solutions to clients in the pharmaceutical and biotechnology space. Lonza offers a large list of products and services from its cell-growth media to in-house production of therapeutics at small, mid, and large-scales for small clinical and biotech companies.
Lonza currently has 37 manufacturing sites world-wide and approximately 14,000 employees.
In recent news, Lonza most notably signed a deal with Moderna to manufacture its COVID-19 mRNA vaccine in 2020. But despite their client-centered focus, Lonza also has active R&D departments that advance the frontier in commercial drugs and therapeutics. In 2020, Lonza spent $5,547 per employee on R&D expenses.
Researchers in the company have been working on developing CDMO solutions to support exosome-based therapeutics as well as researching the efficacy of dry-powder inhalers that deliver bevacizumab to treat lung cancer.
Cellink and Lonza Partner Up
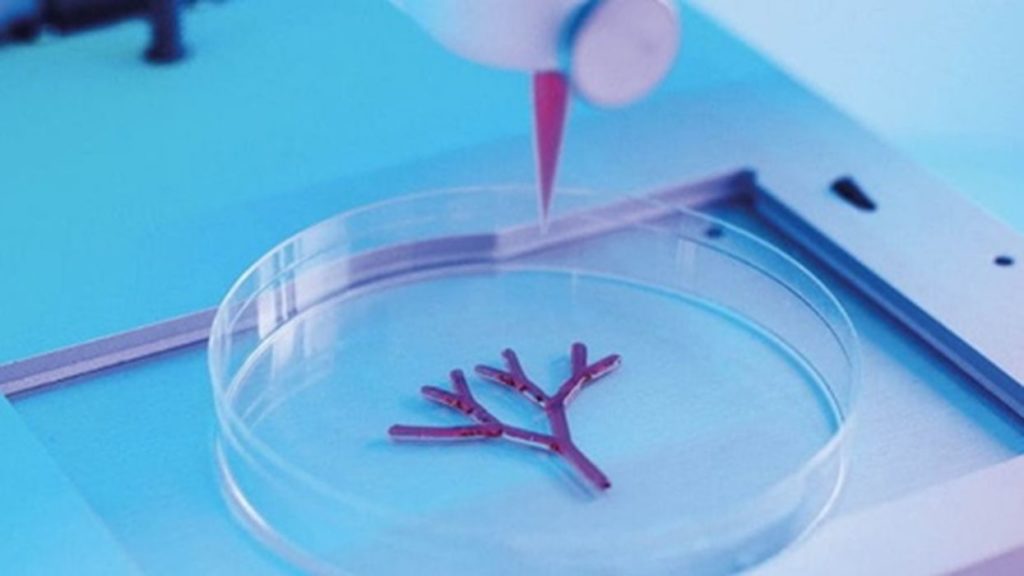
The Cellink-Lonza partnership was announced in June of 2020. Lonza brings to the table its diverse assortment of human-derived primary cells and culture-growth media. Combined with Lonza’s ingredients, the Cellink product line is greatly expanded to accommodate diverse research needs.
Cellink aims to provide high-quality 3D cell culture workflows. This enables cell biologists to work on advanced 3D bioprinting of human tissue samples. This is particularly advantageous as 3D printed cell cultures better model in vivo conditions (Latin for “within the living”) while being handled in an in vitro (Latin for “in glass”) environment. Cellink hopes that its solutions will make animal-testing obsolete. Their product line includes cells, bioinks, and media for liver, heart, and lung tissues.
The Research and Development Tax Credit
The now permanent Research and Development (R&D) Tax Credit is available for companies developing new or improved products, processes and/or software.
3D printing can help boost a company’s R&D Tax Credits. Wages for technical employees creating, testing, and revising 3D printed prototypes can be included as a percentage of eligible time spent for the R&D Tax Credit. Similarly, when used as a method of improving a process, time spent integrating 3D printing hardware and software counts as an eligible activity. Lastly, when used for modeling and preproduction, the costs of filaments consumed during the development process may also be recovered.
Whether it is used for creating and testing prototypes or for final production, 3D printing is a great indicator that R&D Credit eligible activities are taking place. Companies implementing this technology at any point should consider taking advantage of R&D Tax Credits.
Conclusion
For 3D bioprinting to realize its potential, it will require the collaboration of leading multinational biotechnology, chemical, and pharmaceutical companies. The Lonza and BICO-Cellink partnership is a development that is emblematic of the level of collaboration required.

