New research has uncovered previously unrecognized toxicity issues with certain biocompatible resins.
The resins in question are typically used in dental applications. The dental industry has increasingly adopted 3D printing as a solution due to its ability to mass produce customized dental appliances. 3D printed dental applications are so useful that a number of 3D printer manufacturers have been attracted to focus specifically on that industry segment.
The new research, done by Northwestern University, was in a way an accidental discovery. It seems that the researchers were originally intending on scaling up the capability of their previously developed “female reproductive tract on a chip”, which is used for ex vivo research.
To build a new device, they turned to 3D printing, as they felt it could be a useful approach for creating the necessary parts. However, they knew they’d better certify the non-toxicity of the recommended materials before proceeding, as any toxicity could screw up future experimental results.
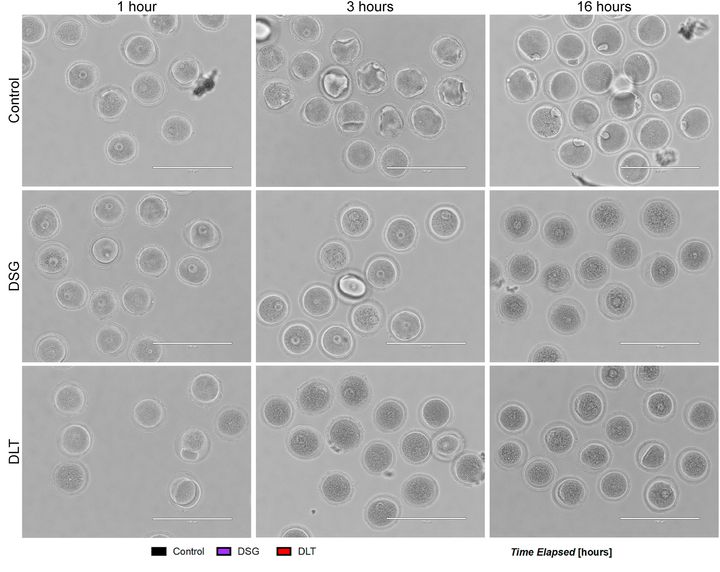
The specific resins involved were Formlabs’ Dental SG and Dental LT Clear, and tested on a Form 2 SLA 3D printer. The paper includes the lot numbers for the resins used in their experiments.
Their toxicity screening involved testing an in vitro mammalian oocyte maturation assay. They found:
“Culture of mouse oocytes in 3DP plates using conventionally treated DSG resin resulted in rapid oocyte degeneration. Oxygen plasma treatment of the surface of printed DSG resin prevented this degeneration, and the majority of the resulting oocytes progressed through meiosis in vitro. However, 57.0% ± 37.2% of the cells cultured in the DSG resin plates exhibited abnormal chromosome morphology compared to 19.4% ± 17.3% of controls cultured in polystyrene. All tested DLT resin conditions, including plasma treatment, resulted in complete and rapid oocyte degeneration.”
Subsequent investigation revealed that Tinuvin 292, used as a light stablizer in Dental LT, had leached out from the prints into the biomaterials, causing the toxicity.
The paper said:
“Severe reproductive toxicity induced by in vitro exposure to these 3D-printed resins highlights potential risks of deploying insufficiently characterized materials for biomedical applications and underscores the need for more rigorous evaluation and designation of biocompatible materials.”
Does this mean that these resins, and potentially other “biocompatible” resins, are not actually biocompatible?
I think the answer is a lot more complex than one might think. It’s easy to read a label that says “Biocompatible” and assume that means the product is compatible with any situation. That’s certainly not the case.
In fact, the label “biocompatible” actually has significant definition behind it, provided by the regulatory agencies. If a manufacturer, such as Formlabs, meets those definitions, then they can use the label. That’s what’s happened here.
If you’ve ever read any material safety data sheets, you’ll understand that the toxicity of a material is highly dependent on the amount, type and time of exposure. A substance that may be declared “safe” is actually safe only within certain usage parameters.
Even “known safe” substances can be toxic if taken in incorrect amounts. I recall the famous proposal by physicist Bernard Cohen, who challenged environmentalist Ralph Nader to consume as much caffeine as Cohen would eat plutonium. Cohen was not taken up on his challenge.
It all depends on what you’re doing and how you’re doing it.
In this case, it may be that the official definition of biocompatibility does not include the scenario proposed by the researchers.
The researchers said:
“Although both DSG and DLT are marketed as ‘biocompatible’ photopolymers, our results demonstrate that the context for biocompatibility is critical.”
The conclusion here is that yes, the resins are biocompatible — but only for the specified use patterns. Always check, and then check again!
Via Science Direct
