
Charles R. Goulding and Preeti Sulibhavi remark on Philips’ transformation into a healthcare-focused company how its engagement in 3D printing activities can help.
After some very difficult problem business years, Philips has evolved from a conglomerate to a healthcare-focused company. The new direction has caught the attention of the Italian Agnelli family which recently purchased 15 percent of the company for US$2.84 billion using their Exor investment vehicle.
Philips had 2022 sales of US$18.78 billion and had 77,233 employees during that year. It used to be a huge multi-product business that included its healthcare businesses.
The Agnelli family owns Ferrari, which is renowned for using 3D printing with their Formula 1 innovation. Philips has extensive experience with 3D printing with their Signify lighting business.
Philips has done remarkable work in the healthcare industry, including 3D printing developments. The new Chief Medical Officer, CMO, Dr. Atul Gupta, has a brand-new vision for the company. More specifically, Gupta leads the management team for Image Guided Therapy (IGT) at Philips and is responsible for clinical strategy as well as lending his voice to product development, IGT support systems, and IGT devices.
He also supports Philips’ efforts in clinical education, new business development, office-based labs and managed services, and medical affairs. Gupta coordinates the visions of various businesses of Philips as well, to seamlessly integrate technologies to advance minimally invasive procedures and outcomes.
Gupta’s vision, in alignment with Philips’ global strategy, is to improve the health outcomes for 2.5 billion people by the year 2030.
Philips and 3D Printing
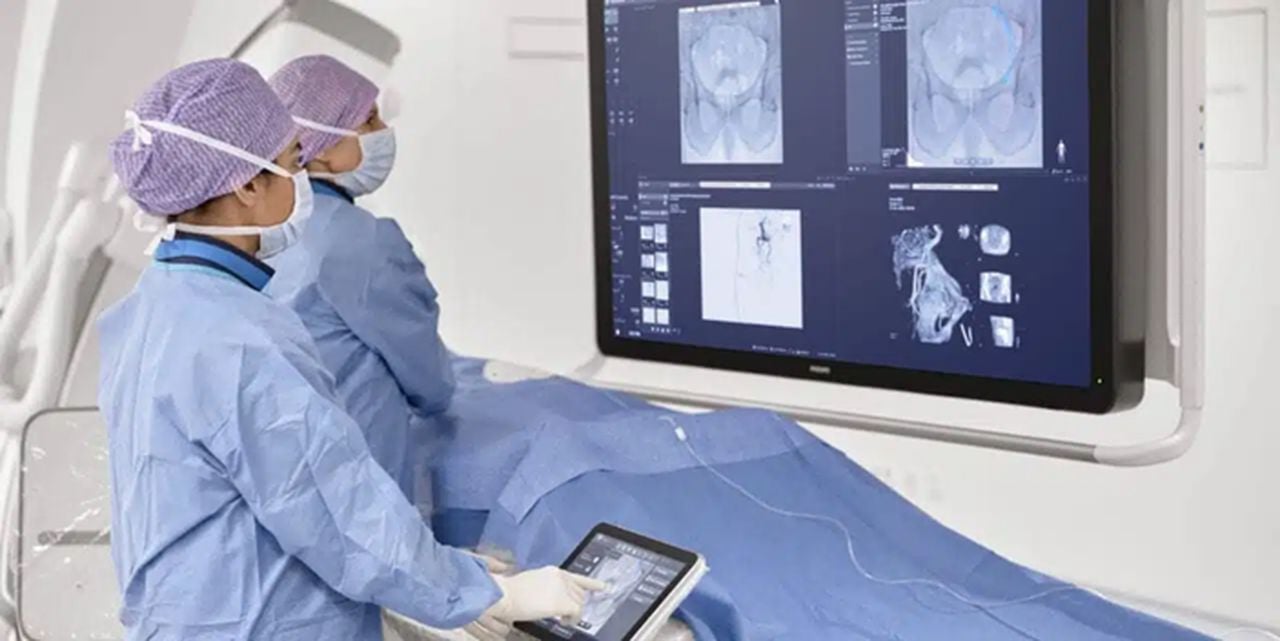
In the medical field, radiologists use images of the body to diagnose and treat diseases and injuries. Advanced 3D modeling enhances radiologists’ ability to review complex, multi-disciplinary medical cases. In terms of health technology, Royal Philips (the health technology business segment of the global giant) is no stranger to 3D printing.
To assist radiologists with the imaging tools they need to successfully and substantially improve health outcomes for patients, Philips has teamed up with 3D Systems and Stratasys for 3D printing expertise in creating models that visualize the effects of various diseases on the human body. This can lead to improved quality and personalization of treatment and medical care for patients with diseases like cancer and orthopedic illnesses.
For example, Stratasys’ unique PolyJet-based, full-color, multi-material 3D printers enable medical professionals to innovate and achieve superior levels of patient care. Interfacing with Philips, customers can now rapidly design, order, and produce 3D printed anatomical structures on-demand from Stratasys Direct Manufacturing.
In addition, Philips offers end-users the opportunity and ability to create models in its IntelliSpace Portal 10 (a platform that enables clinicians to render and edit anatomical models that reflect customized patient anatomies) and transfer the files to 3D vendors without leaving their medical office or hospital.
In 2021, the US Food and Drug Administration (FDA) granted 510(k) clearance to Philips’ SmartCT application software, a key component of its Image Guided Therapy System-Azurion, which provides clinicians with CT-like 3D images. Advancing 3D imaging and visualization is enhanced with SmartCT software that has applications for various medical interventions including angiography, neurology, soft-tissue imaging, and catheter navigation.
Prior to this, Philips had received FDA clearance for its Ingenia Elition 3.0T MR device that enables physicians to perform exams 50 percent quicker and increase diagnostic confidence for more effective treatment.
The investment vehicle for the Agnelli family investment in Philips is Exor, which has a diversified portfolio. In 2019, it merged with the firm Stellantis (FCA and PSA) and now, Exor owns 14.5% of the auto industry leader. We have covered the 3D printing activities underway at Stellantis previously. Philips can now leverage the 3D printing advancements at Stellantis and apply those technologies to the medical field since they are both assets in Exor’s portfolio.
The Research & Development Tax Credit
The now permanent Research and Development (R&D) Tax Credit is available for companies developing new or improved products, processes and/or software.
3D printing can help boost a company’s R&D Tax Credits. Wages for technical employees creating, testing and revising 3D printed prototypes can be included as a percentage of eligible time spent for the R&D Tax Credit. Similarly, when used as a method of improving a process, time spent integrating 3D printing hardware and software counts as an eligible activity. Lastly, when used for modeling and preproduction, the costs of filaments consumed during the development process may also be recovered.
Whether it is used for creating and testing prototypes or for final production, 3D printing is a great indicator that R&D Credit eligible activities are taking place. Companies implementing this technology at any point should consider taking advantage of R&D Tax Credits.
Conclusion
When former multi-unit businesses get focused, they can concentrate on one core business for product design and volume manufacturing. This is exactly the right scenario to support the broader use of 3D printing for Philips and its affiliates.
