The US FDA has been engaged with other parties to assist in the use of community 3D printing resources to confront the COVID-19 crisis.
The organization issued a statement recently updating their progress, as they are a key partner with several other organizations that are attempting to provide direction to the 3D printing community. Many in the community in the US are keenly interested in helping by providing production capability for needed items, but these individuals almost always require assistance.
Previously we described how one operation in Huntsville, Alabama self-organized an impromptu production operation for collection, sterilization, packaging and delivery of needed COVID-19 parts. While they organized the production, there was also the question of “what to produce”.
This has been a difficult question to answer during the crisis, as we have all been beset with countless designs for all kinds of parts. Generally we can’t tell a) whether the part functions as it is intended, b) whether the part’s functions are suitable for medical environments, c) whether it’s actually needed, and d) who, exactly needs them.
Organizing things at a level necessary to solve such questions is usually beyond modest local groups, and that’s where the big guys, like the FDA and NIH, come in. They have developed a process to coordinate all of these factors in one place, as best they can.
NIH 3D Model Process
Here’s a graphic of their overall process:
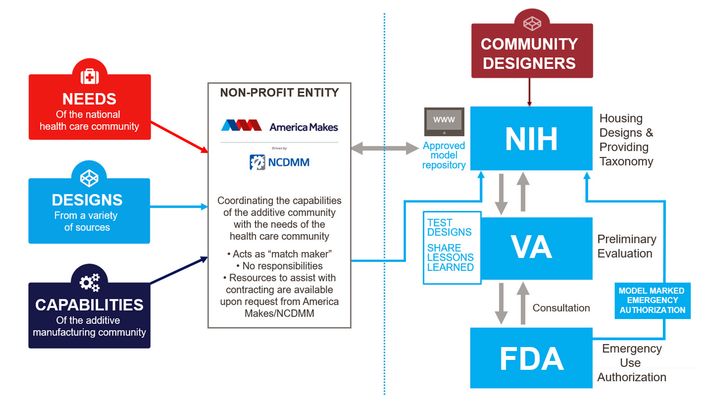
As we can see in this workflow, potentially useful designs are submitted through America Makes, which forwards them to the NIH. The VA provides a preliminary evaluation, and the final certification for emergency use is provided by the FDA.
This then allows the 3D model to be placed in the official NIH “approved model repository”, where participants can draw from when items are to be produced via 3D printing.
NIH 3DPX
As of today, the NIH 3D Print Exchange (NIH 3DPX) hosts 31 designs certified for “clinical use”, and 28 for “community use”, where anyone can make use of the products. In addition to the certified 3D models, there are hundreds of other proposed items, which NIH 3DPX labels as “Prototypes”. There are also 8 3D models for COVID-19 test swabs.
Most of the items in the inventory seem to be simple objects such as face shields, mask-related items, door openers and ear savers.
Curiously, there are 33 items labeled “WARNING” by NIH 3DPX. What does that mean? They say:
“This device requires FDA approval or it may have design flaws that introduce safety risks. It has not been assessed for safe use.”
That could mean it just hasn’t been looked at yet, or it has been examined and found to be flawed. The items in this category seem to be more advanced items like respirator valves, etc. In any event, they likely should be avoided until they are approved. It may be that “bad” items are left in the repository to ensure that everyone knows they are not approved.
And that’s the point of this repository: to provide a single platform for US participants to obtain good advice on which 3D models to produce. Without such a resource we’d be swimming in questionable 3D prints, as was the case much earlier in the crisis.
If you’re intending on producing emergency items for the crisis with your 3D printing equipment, the NIH 3DPX is probably a good place to start looking for approved 3D models. Note that while the site is intended for US participants, the 3D models have been made freely downloadable by anyone, anywhere for similar activities in other countries. Thanks, NIH!
