Charles R. Goulding and Preeti Sulibhavi highlight Eli Lilly’s remarkable growth and groundbreaking advancements in pharmaceuticals, from Alzheimer’s treatment to innovative 3D printing drug delivery.
fda
BMF’s UltraThineer Earns FDA Clearance for Cosmetic Veneers
Boston Micro Fabrication has received an important approval for a new material.
3D Systems Achieves 510(k) Clearance for PEEK Cranial Implants
The FDA has approved the 3D printed, patient-specific VSP PEEK Cranial Implant solution.
3D Printing’s Role in Custom-Fit Hearing Aids and Dementia Risk Reduction
Charles R. Goulding and Preeti Sulibhavi discuss how companies in the medtech industry can leverage 3D printing to enhance customization and fit of hearing aids.
3D Printing in Medicine: Philips’ Partnerships with 3D Systems and Stratasys
Charles R. Goulding and Preeti Sulibhavi remark on Philips’ transformation into a healthcare-focused company how its engagement in 3D printing activities can help.
Navigating Regulatory Challenges: Ricoh’s Journey in 3D Healthcare Printing
Ricoh seems to have found a promising niche in the 3D print world: anatomical models.
FDA Approves Over-the-Counter Hearing Aids and 3D Printing Can Help
Charles R. Goulding and Preeti Sulibhavi look at the potential for use of 3D printing to produce hearing aids on demand.
DyeMansion’s VaporFuse Process Breaks the Food Barrier
DyeMansion announced a new food safe capability for their VaporFuse process, and several corporate advancements.
President Biden’s Executive Order To Improve Hearing Aid Availability And 3D Printing
Charles R. Goulding and Arianna Coger listen to ways 3D printing continues to innovate in hearing aid production.
Where You Can Find Certified COVID-19 3D Models
The US FDA has been engaged with other parties to assist in the use of community 3D printing resources to confront the COVID-19 crisis.
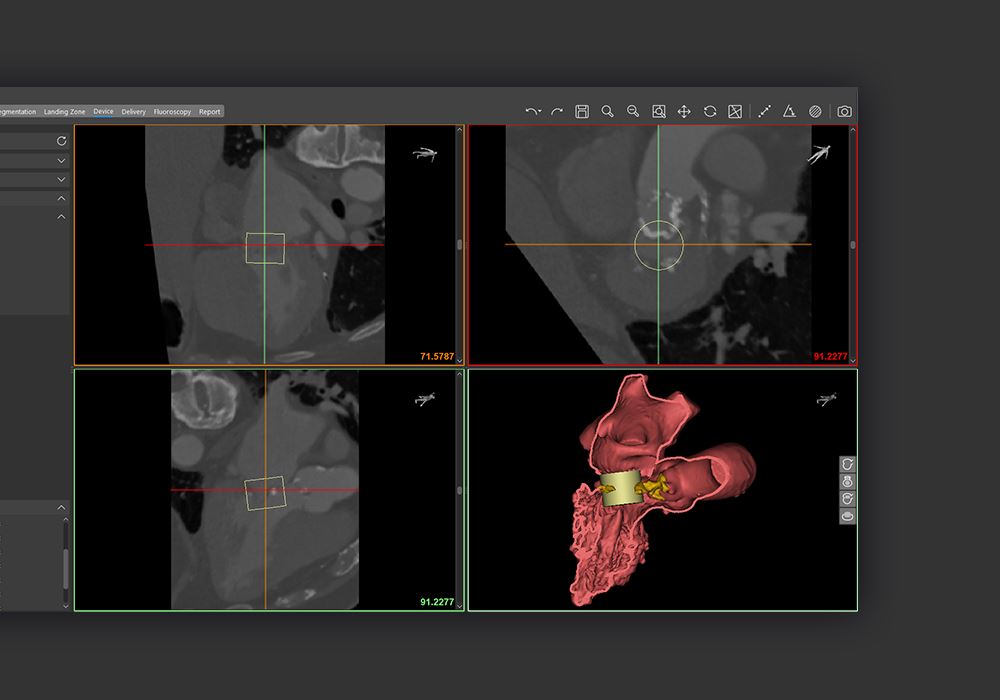
Materialise Enlightens Medical Care With FDA-Approved Cardiovascular Software
Materialise has received FDA clearance for its cardiovascular planning software.
FDA Awards Grants to Bioprinting Institutions
Charles Goulding and Rafaella July of R&D Tax Savers explore the US FDA’s grants forwarding work in bioprinting.
Materialise Mimics inPrint Certification Program Clears Ultimaker and Formlabs for Medical 3D Printing
Materialise’s announcement today of FDA-cleared medical model 3D printing encompasses vat polymerization and material extrusion technologies in addition to PolyJet.
FDA-Cleared Medical 3D Printing with Stratasys and Materialise
3D printing medical models offers benefits to patients and those providing their care with highly accurate patient-specific anatomies able to be held, examined, and practiced on.
What is a 510(k) Clearance and Why do 3D Print Companies Need it?
Occasionally I will see companies announce they’ve received a “501(k) clearance”. What is that and why is it important?