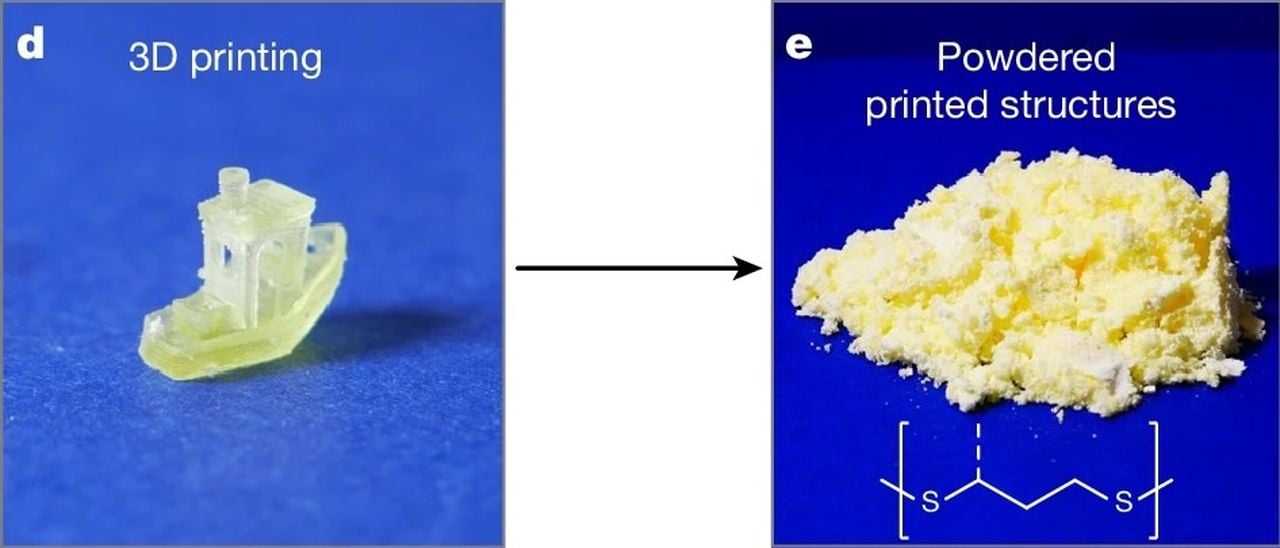
Researchers have developed a new type of resin for 3D printing that can be fully recycled.
Recycling of resin 3D printer output has been essentially impossible. That’s because the resulting prints are thermosets, not thermoplastics.
Thermoplastics have the interesting property of softening when heated, and then re-solidifying when cooled. That’s how all FFF 3D printers work: the hot end heats the material, which then cools after extrusion.
Thermoplastics are relatively easy to recycle: just heat them up and shape them into new forms.
Thermosets are not like that at all. They polymerize (solidify) into a shape and that’s it. There is no way to unlink the molecules that are now bound together in a rigid manner. Because of this any 3D prints produced on a resin 3D printer will ultimately go to the landfill. There is no possibility of recycling the material as the acrylic or epoxy chemistry prevents this.
That may all change with a brilliant development from researchers at the University of Birmingham, who concocted a new recipe for resin that not only is manufactured from bio-sourced feedstock, but also can be almost fully recycled in a closed loop process.
How could this work? They explain in their recent paper:
“Here we describe a photopolymer resin platform derived entirely from renewable lipoates that can be 3D-printed into high-resolution parts, efficiently deconstructed and subsequently reprinted in a circular manner. Previous inefficiencies with methods using internal dynamic covalent bonds to recycle and reprint 3D-printed photopolymers are resolved by exchanging conventional (meth)acrylates for dynamic cyclic disulfide species in lipoates. The lipoate resin platform is highly modular, whereby the composition and network architecture can be tuned to access printed materials with varied thermal and mechanical properties that are comparable to several commercial acrylic resins.”
The key here seems to be the replacement of acrylates with liopates.
The researchers tested the resin by 3D printing typical benchmark structures, including a #3DBenchy, and found they were able to reliably print features as small as 0.100mm, equaling the capability of most of today’s resin 3D printers.
The resin was sensitive enough that they recommend adding an opaquing agent to the material so that light cannot penetrate deeper than the intended layer depth.
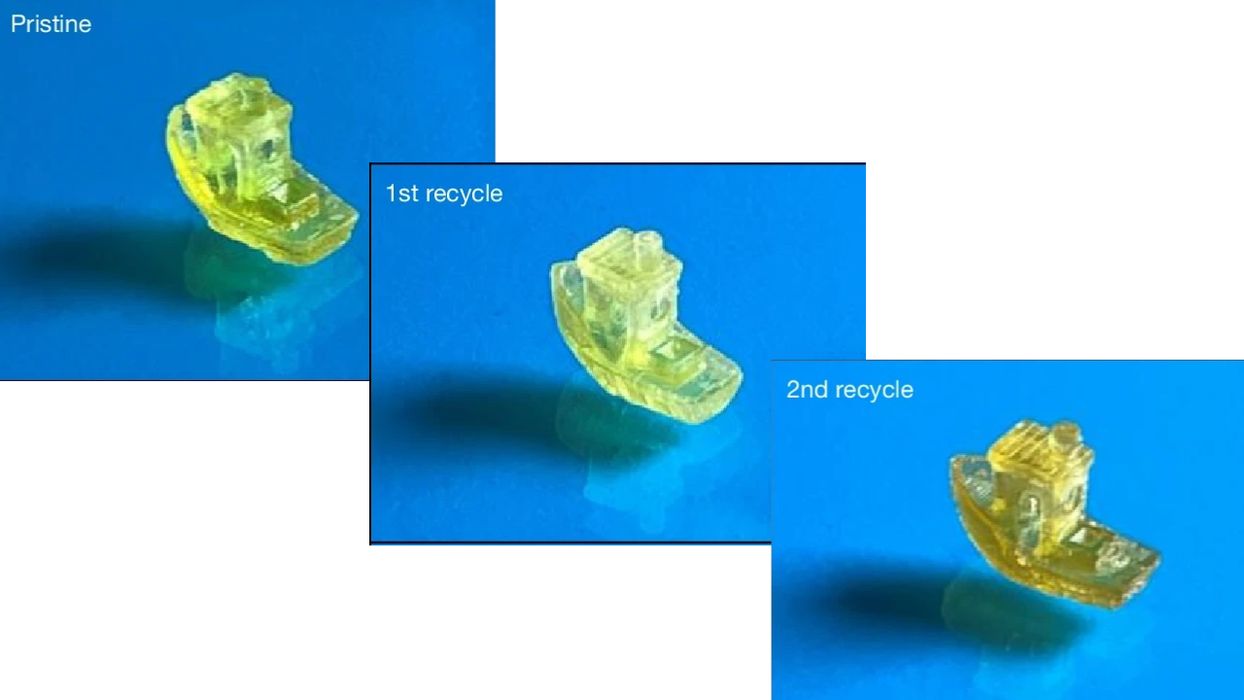
That’s all good, but the big test was whether this material could be recycled. They attempted several processes to break down the material, including use of a green solvent, 80C heat, nitrogen atmosphere, and other chemicals. They found that some of these approaches worked, and achieved high rates of depolymerization. They explain:
“Size-exclusion chromatography (SEC) analysis of the recycled resin showed that it had a similar molecular weight profile to the original resin, although a small amount of oligomeric product was observed, which indicates that the depolymerization was not 100% effective. Nuclear magnetic resonance (NMR) spectroscopic analysis of the recycled resin enabled calculation of the recycled resin purity of 85 mol%, with the minor component being an oligomeric species. The ratio of MenLp1 to IsoLp2 in the recycled resin was calculated to be a 1 to 1.53 mol ratio (31:69 wt%), which is within experimental error of the as-synthesized mixture before polymerization (printing). Together this indicates that the overall resin composition was comparable to the initial formulation, thus demonstrating that the resin was both 3D printable and could be depolymerized efficiently.”
This is important work, and I am hoping it will lead to widespread use of fully recyclable resins in 3D printing.
However, there are plenty of questions. Some that come to mind are:
- What are the engineering properties of the parts produced with this resin? Would they be suitable for many applications?
- How do these properties change after multiple recycle sequences?
- Is it possible to use additives in the resin, for example to perform ceramic prints?
- How complex and expensive are the recycling processes? If they are too expensive, they will not be adopted.
And a final question: which resin manufacturers will license this technology and commercialize it?
Via Nature (Hat tip to Benjamin)
